
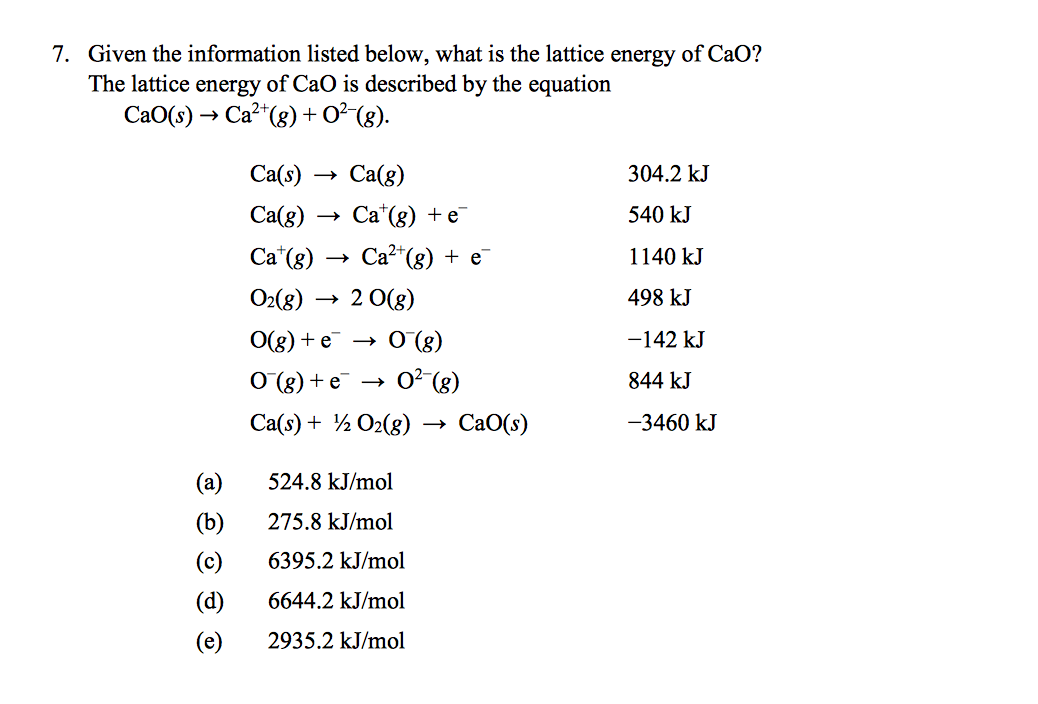

We begin with the elements in their most common states, Cs( s) and F 2( g). The lattice energy of nearly any ionic solid can be calculated rather accurately using a modified form of Equation 4.2.1: (4.2.1) U k Q 1 Q 2 r 0, w h e r e U > 0. \): The Born-Haber cycle shows the relative energies of each step involved in the formation of an ionic solid from the necessary elements in their reference states. 3 Computation of Lattice Energies and Crystal Structures The calculation of the lattice energy of an ordered crystal with a known structure by the atom-atom.


 0 kommentar(er)
0 kommentar(er)
